
Without proper EUDAMED registration and SRN, manufacturers cannot legally place their devices on the EU market.
The EAR ensures smooth registration, and quick communication with Authorities.
EU Representative for EUDAMED and UDI Submission Services
An EU Authorized Representative (EAR/EC REP) is a natural or legal person based in the European Union (EU) who is officially designated by a non-EU manufacturer to act on their behalf regarding compliance with EU medical device regulations. Since manufacturers outside the EU cannot directly place devices on the EU market, the EC REP serves as the regulatory link between them and EU authorities, including Competent Authorities and Notified Bodies. Below are the legal basis wherein an EAR is mandated.
-
MDR 2017/745 (Medical Devices Regulation)
-
IVDR 2017/746 (In Vitro Diagnostic Regulation)
Both regulations clearly mandate the appointment of an Authorized Representative for non-EU manufacturers.
Key Responsibilities of the EU Representative
- Ensure conformity assessment procedures are carried out by the manufacturer and also verify that the manufacturer has drawn up the EU Declaration of Conformity and Technical Documentation.
-
Serve as the point of contact for EU Competent Authorities and Notified Bodies. Cooperate in case of inspections or product recalls.
-
Ensure manufacturer and device registration in the EUDAMED database. Obtain the Single Registration Number (SRN).
-
Forward serious incidents, field safety corrective actions (FSCA), and complaints to the manufacturer. Support post market surveillance (PMS) activities.
-
Keep copies of the Technical Documentation, CE certificates, and Declarations of Conformity available for inspection for at least 10 years (15 for implantable devices) after the last device was placed on the market.
- The EC REP name, address, contact info and logo must appear on device labelling, packaging, and instructions for use (IFU).
EUDAMED Registration
EUDAMED (European Database on Medical Devices) is the central European database established under MDR 2017/745 and IVDR 2017/746. It is designed to improve transparency, traceability, and market surveillance for medical devices and IVDs in the EU. EUDAMED has six modules but not mandatory for all manufactures. EUDAMED will be completely ruled out by Sep 2027.
-
Actor Registration (Manufacturers, Authorized Representatives, Importers, etc.)
-
UDI/Device Registration
-
Notified Bodies & Certificates
-
Clinical Investigations / Performance Studies
-
Vigilance & Post-Market Surveillance (PMS)
-
Market Surveillance
The first and mandatory step for a non-EU manufacturer is Actor Registration, which leads to the issuance of a Single Registration Number (SRN). This SRN is essential for device registration, Notified Body submissions, and communication with authorities.
EUDAMED Registration Process
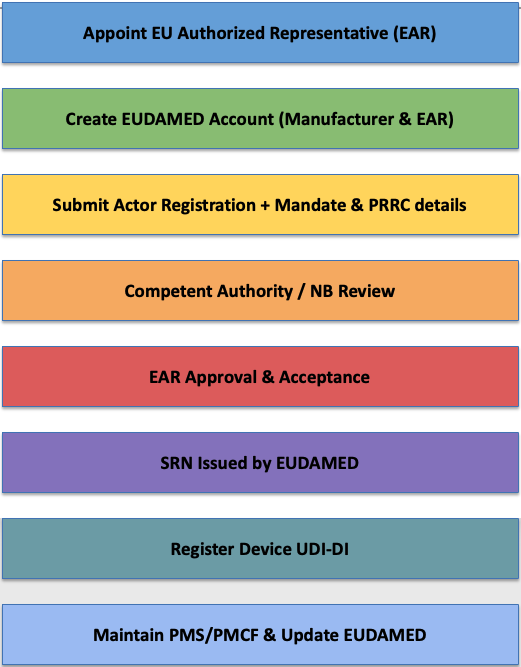
EU Rep and EUDAMED related FAQ’s
Yes. According to MDR and IVDR requirements, the EC REP’s name and address must be clearly visible on the product labeling, outer packaging, or accompanying documents, allowing EU authorities to easily identify the representative.
Yes, we provide full support for UDI implementation and registration in compliance with EU MDR 2017/745 and IVDR 2017/746 requirements. Our team assists manufacturers in assigning and structuring UDI-DI and UDI-PI codes based on the device classification and intended use. We also help in ensuring proper placement of UDI on labeling, packaging, and Instructions for Use (IFU), as well as preparing for UDI module submission once it becomes fully functional within EUDAMED.
Our services cover:
-
UDI-DI and UDI-PI structure guidance
-
Compliance with GS1, HIBCC, or ICCBBA issuing agencies
-
UDI label and packaging review
-
Preparation for EUDAMED UDI/Device registration module
-
UDI-related documentation for your Technical File and CE submission
By integrating UDI properly, we help ensure device traceability, reduce market delays, and support full regulatory compliance across the EU.
Absolutely. In addition to EC REP services, we offer end-to-end regulatory consulting, including Notified Body selection, technical file preparation, and gap analysis to support your CE Marking journey efficiently.
Yes. If your company is based outside the EEA and wishes to market medical devices or IVDs in Europe, it is legally required to appoint an EC REP. This representative’s name and address must appear on the product labeling, outer packaging, or Instructions for Use (IFU).
We offer fast onboarding, typically within 1–2 working days upon receipt of the necessary documentation and agreement. We also guide you through label updates and document handovers to ensure a smooth transition.
As part of our commitment to regulatory compliance and risk management, we recommend that all non EU manufacturers have valid Product Liability Insurance when appointing us as their European Authorized Representative (EC REP). While it is not a legal requirement under MDR/IVDR for the EC REP to request proof of insurance, many Competent Authorities and Notified Bodies may inquire about the manufacturer’s liability coverage during inspections or audits. Having proper insurance also demonstrates due diligence and protects your business in the event of product-related claims in the European market. We do not provide insurance ourselves, but we can guide you on what to include based on your device risk class and market exposure.

