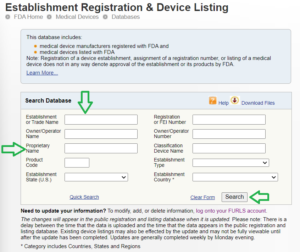
ESTABLISHMENT REGISTRATION (FDA MEDICAL DEVICE REGISTRATION)
The manufacturers, initial exporters, initial importers, labeller, reprocessor, specification developer, US manufacturer for the export only medical device and contract sterilizers of facilities intended for use in the United States are required to register with the FDA on an annual basis. Establishment registration is the term for this procedure (Title 21 CFR Part 807).
All establishments that are required to register with the FDA must also specify the devices they manufacture and the operations they do on those devices. majority of the device needs 510k, De Novo, PMA, PDP, HDE number prior to listing.
MEDICAL DEVICE FDA REGISTRATION AND LISTING
The FDA requires owners or operators of establishments involved in the manufacturing and distribution of medical devices intended for use in the United States to register with the agency on an annual basis. The annual medical device facility registration fee for the fiscal year 2022 is $5672
Domestic Manufactures or Initial Importers
Foreign Manufactures
Foreign Manufactures
MEDICAL DEVICE LISTING
The process of Listing is explained below.
Check facility DUNS number
FURLS account creation and FDA fee payemnt
Identify the device code and regulation number
Confirm the device name and trade name along with models
Receipt of FDA fee payment confirmation
Verify Registration and Listing details in FDA Website
FDA US AGENT
Foreign manufactures and exporters interested in selling medical devices in the USA can approach us. Our extensive knowledge of FDA regulations will aid in a smooth and efficient registration process. We the US Agents awake and answer any time when authorities ask for information. In addition to our US office, we have offices in Germany, India, Malaysia, UK and Vietnam.
We are not just like others, we have a big team of device experts, consultants and project managers who can handle any type of service to your organization.
The US Agent Service is vital for FDA medical device registration of foreign medical and in-vitro diagnostic devices manufacturers and initial exporters.
BEST PRICE OFFER
Appointing the US Agent, Making a payment to FDA, Complete the registration and Listing all can be completed in just 2-3 days.
24X6 Chat support
6 days working and processing files
Economic pricing and polite staff
Annual US Agent Fees: $449
Device Listing one-time fee: $100
Annual US Agent Fees: $549
Device Listing one-time fee: $100
Annual US Agent Fees: $649
Device Listing one-time fee: $150
Review and Guide device Label $950
Timeline: 7 Working days
FDA REGISTRATION FORM
Medical Devices
Registration and Listing can be completed in 3-4 working days. This is not an FDA form.
TRANSFER OF US AGENT
MEDICAL DEVICES
We are happy to act as US Agent for any non-US manufacturers. Please bring us the FDA FURLS account details to update the Agent Infomation.