
Accurate IVD Classification — The First Step to IVDR Compliance
Classify Right. Certify Faster.
IVDR Classification Guide | EU IVD Device Risk Classes Explained
The IVDR went into effect on May 26, 2017, after a five-year transition period. Manufacturers and developers will have to find new ways to sell their products, and suppliers will have to alter old IVDs to meet new standards. One of the most significant changes in the new legislation is that IVDs are now classified using “risk-based standards,” rather than a pre-determined list of devices.
The following are the major features of the new risk-based classification:
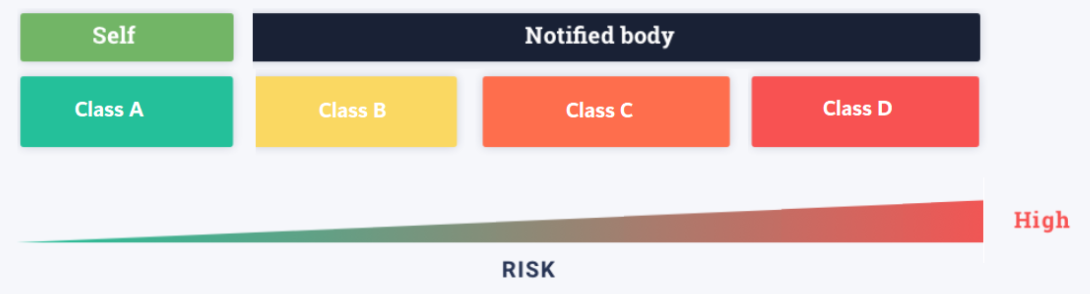
Key IVDR Classification Rules Explained
[qepw_pdf_viewer file=”https://www.reghelps.com/wp-content/uploads/2025/08/md_mdcg_2020_guidance_classification_ivd-md_en.pdf”]
service related FAQ’s
Based on the number of models and varients the timeline may change. usually the timeline for class B is approx 8-10 montsh and Class D is 14 months.

